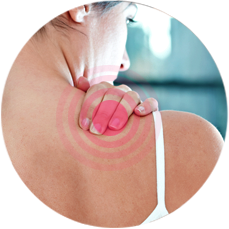
Pain Relief



Reduction of unwanted fat with body contouring Innovative design and intuitive user interface
Safe, easy, while being applied to a wider area, shorter time Painless treatment


Low-Level Laser Therapy (LLLT) is commonly used in medical applications, but scientific studies of its efficacy and the mechanism by which it causes loss of fat from fat cells for body contouring are lacking. This study examined the effectiveness and mechanism by which 635-680nm LLLT acts as a non-invasive body contouring intervention method.

Pain Relief

Hair Growth
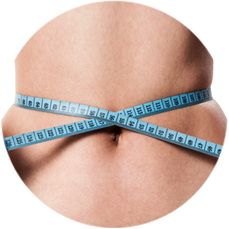
Body Contouring

Tinnitus



Non invasive &
Painless treatment

Various targeted
area treatment

Safe &
Comfortable procedure

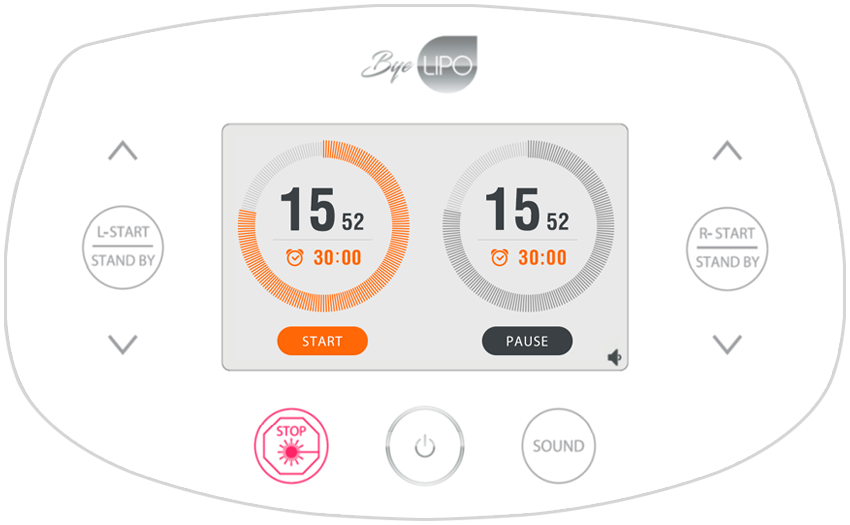
Easy self controlling &
Smart system

Excellent result
in a short time
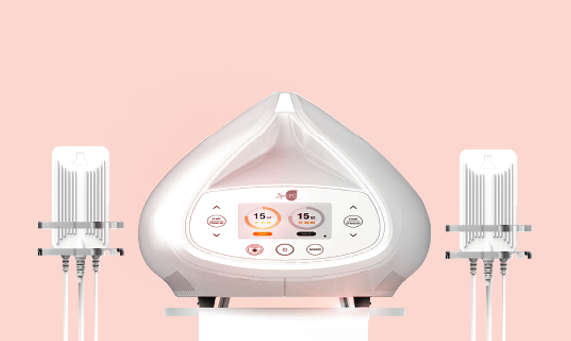
Total of 12 probes for customized area treatments
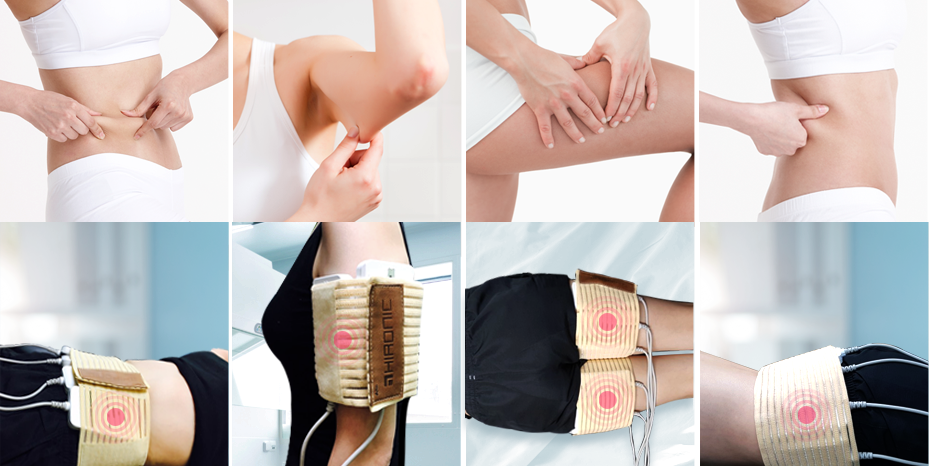
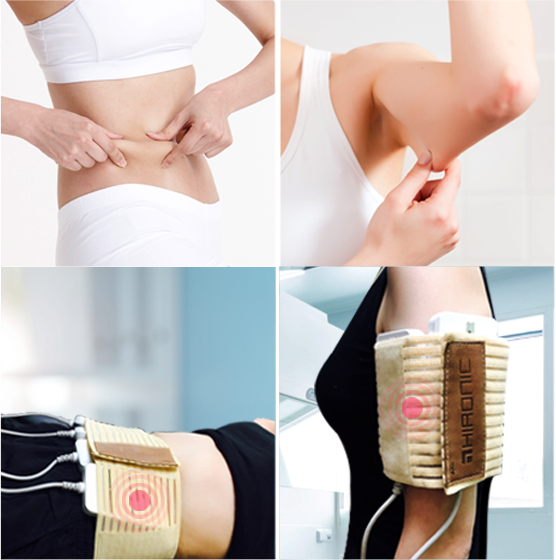
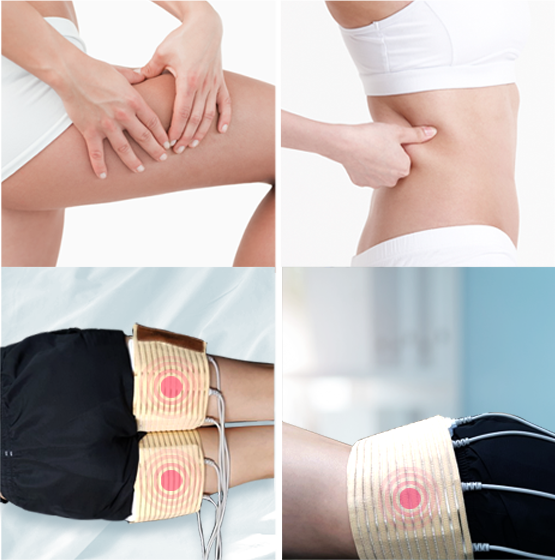
8 Multi probes and 4 EN probes
Total of 12 probes
Excellent result in a short time



Non invasive and painless treatment for customers between all ages

Safe and comfortable procedure for customers who may fear other treatments
Source Type
AlGanlnP(Diode)
Wavelength
658nm
Output Power
40mW / beam + 5%
Multi Probe
8 channel (8EA LD each)
En Probe
4 channel (1EA LD each)
Operating Mode
Continuous wave
Power Supply
100-240Vac, 50/60Hz
Power Consumption
Max 100VA
Display
5 inch TFT LCD
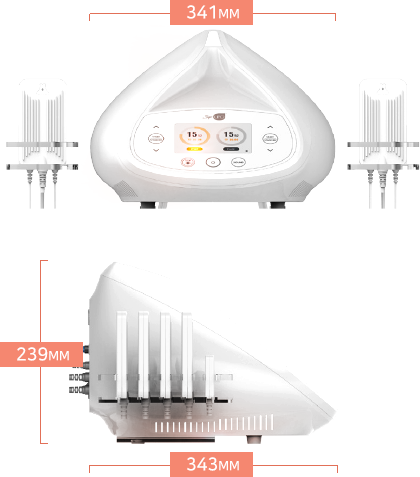
dimensions (mm)
341(W)x343(D)x239(H)
Wieght
7.5Kg


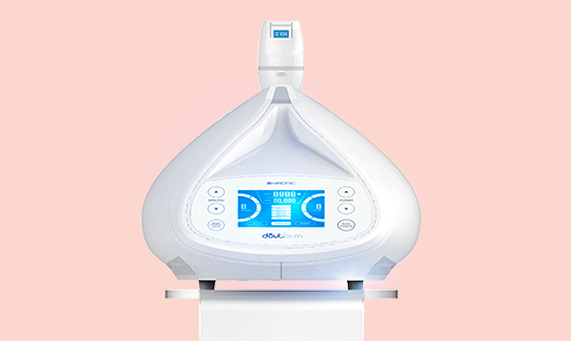


Focused Ultrasound
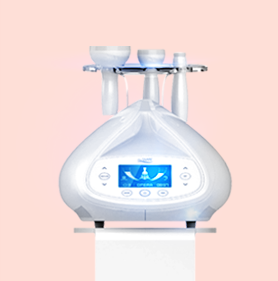
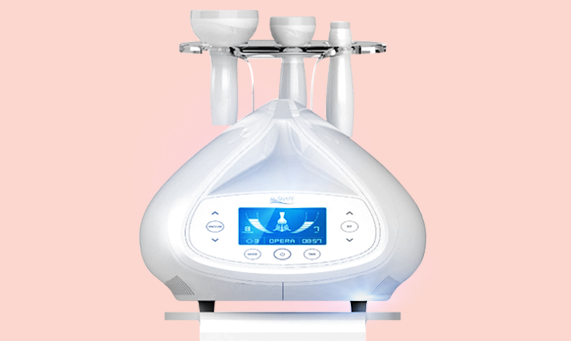


Multipolar RF + Vacuum + LED
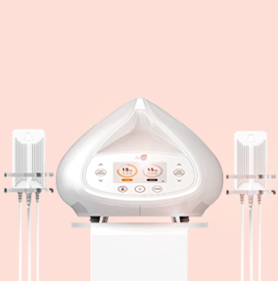

Laser Therapy

Dubai Derma 2019
Location : International Convention
Date : March 18 – March 20
HIRONIC participated at Dubai Derma 2019!
Regarding the exhibition, more than 200 visitors visited our booth and got many inquiries from 25 countries. Thanks to visitors, we could finish this exhibition very successfully, and it was a good opportunity to know the market trend and needs in the Middle East region and promote our new products such as Plasonic and Slimus. We sincerely hope to see you at Dubai Derma 2020 again.
Location : International Convention
Date : March 18 – March 20

HIRONIC participated at Dubai Derma 2019!
Regarding the exhibition, more than 200 visitors visited our booth and got many inquiries from 25 countries. Thanks to visitors, we could finish this exhibition very successfully, and it was a good opportunity to know the market trend and needs in the Middle East region and promote our new products such as Plasonic and Slimus. We sincerely hope to see you at Dubai Derma 2020 again.





KIMES 2019
Location : COEX, Seoul, Korea
Date : March 14 – March 17
HIRONIC participated at KIMES 2019!
HIRONIC successfully finished KIMES 2019 (35th Korea International Medical & Hospital quipment Show) held at the COEX Exhibition Center in Seoul from March 14th - 17th. KIMES is the largest medical equipment show in Korea and more than 70,000 visitors including 4,143 foreign visitors attended. Taking this opportunity, HIRONIC exhibited its main flagship products such as DOUBLO GOLD, MICOOL, SLIMUS, PLASONIC, ULTRA VERA, Q-FIT, and A-FIT. We were pleased to meet the medical professionals and share ideas with existing and potential partners. We are already looking forward to KIMES 2020 and welcome your visit.
Location : Seoul, Korea
Date : March 14 – March 17

HIRONIC participated at KIMES 2019!
HIRONIC successfully finished KIMES 2019 (35th Korea International Medical & Hospital quipment Show) held at the COEX Exhibition Center in Seoul from March 14th - 17th. KIMES is the largest medical equipment show in Korea and more than 70,000 visitors including 4,143 foreign visitors attended. Taking this opportunity, HIRONIC exhibited its main flagship products such as DOUBLO GOLD, MICOOL, SLIMUS, PLASONIC, ULTRA VERA, Q-FIT, and A-FIT. We were pleased to meet the medical professionals and share ideas with existing and potential partners. We are already looking forward to KIMES 2020 and welcome your visit.







ADD 2019
Location : WASHINGTON, D.C.
Date: March 1 - March 3
HIRONIC participated at AAD 2019!
HIRONIC attend AAD 2019 (American Academy of Dermatology) which is held at the Washington Convention Center from March 1st - 3rd, 2019. More than 18,000 doctors attended and, with this opportunity HIRONIC displayed its prime products such as SLIMUS, PLASONIC, Q-FIT, and A-FIT(USA FDA approved). We were glad to meet our current partners and also new potential partners and enjoyed the meetings with many medical professionals. HIRONIC is already looking forward to AAD 2020 to be held in Denver and welcomes your visit
Location : WASHINGTON, D.C.
Date: March 1 - March 3

HIRONIC participated at AAD 2019!
HIRONIC attend AAD 2019 (American Academy of Dermatology) which is held at the Washington Convention Center from March 1st - 3rd, 2019. More than 18,000 doctors attended and, with this opportunity HIRONIC displayed its prime products such as SLIMUS, PLASONIC, Q-FIT, and A-FIT(USA FDA approved). We were glad to meet our current partners and also new potential partners and enjoyed the meetings with many medical professionals. HIRONIC is already looking forward to AAD 2020 to be held in Denver and welcomes your visit








IMCAS 2019
Location : Paris, France
Date : January 31 – February 2
HIRONIC participated at IMCAS 2019!
IMCAS 2019 was another successful exhibition for HIRONIC! We had meaningful meetings with various medical professionals in field, and would like to thank everyone who visited our booth to share valuable time together. Not only that, we had a lecture about Doublo Gold and Plasonic, presented by Dr. Sabatier (Dermatologist, France) to provide clinical information about devices for users.
Location : Paris, France
Date : January 31 – February 2

HIRONIC participated at IMCAS 2019!
IMCAS 2019 was another successful exhibition for HIRONIC! We had meaningful meetings with various medical professionals in field, and would like to thank everyone who visited our booth to share valuable time together. Not only that, we had a lecture about Doublo Gold and Plasonic, presented by Dr. Sabatier (Dermatologist, France) to provide clinical information about devices for users.



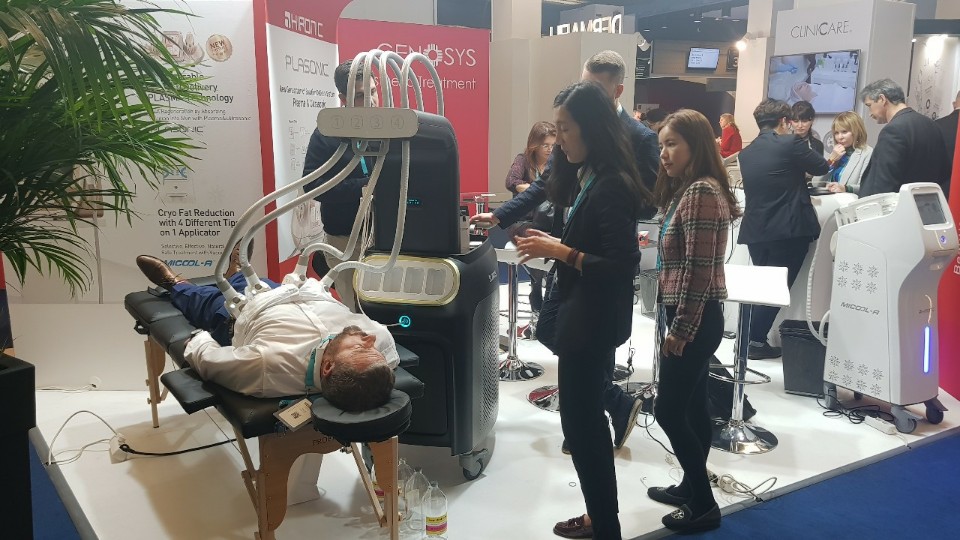

EADV 2018
Location : Paris, France
Date : September 12 – September 16
HIRONIC will participate at EADV 2018!
EADV2018 will provide the best platform for all participants to boost and update their knowledge in dermatological science and technology through a variety of courses, workshops and lectures. Join HIRONIC to see innovative devices which includes our newly launched SLIMUS.
Location : Paris, France
Date : September 12 – September 16

HIRONIC will participate at EADV 2018!
EADV2018 will provide the best platform for all participants to boost and update their knowledge in dermatological science and technology through a variety of courses, workshops and lectures. Join HIRONIC to see innovative devices which includes our newly launched SLIMUS.



FACE 2018
Location : QEII Centre, London
Date : JUNE 14 – JUNE 16
HIRONIC participated at FACE 2018!
Hironic was able to successfully attend FACE 2018, UK’s premiere scientific conference featuring the latest clinical information, practical tips and updates in the field aesthetic treatment. It was our second launching show for SLIMUS and thankfully many doctors and professionals were interested in SLIMUS, and had done many demonstration treatments. For FACE 2018, HIRONIC displayed Doublo Gold, Micool-A, and SLIMUS. Once again, we highly appreciate your visit. See you soon!
Location : QEII Centre, London
Date : JUNE 14 – JUNE 16

HIRONIC participated at FACE 2018!
Hironic was able to successfully attend FACE 2018, UK’s premiere scientific conference featuring the latest clinical information, practical tips and updates in the field aesthetic treatment. It was our second launching show for SLIMUS and thankfully many doctors and professionals were interested in SLIMUS, and had done many demonstration treatments. For FACE 2018, HIRONIC displayed Doublo Gold, Micool-A, and SLIMUS. Once again, we highly appreciate your visit. See you soon!
하이로닉 영문 웹사이트로 이동합니다.
글로벌 웹사이트에는 수출용 제품으로
대한민국에서 허가하지 않은 장비 및 서비스
내용을 포함하고 있습니다. 해당 내용은
대한민국의 의료기기 광고 심의, 규제를 받지 않습니다.
It goes to the HIRONIC English website.
The Global Web Site contains equipment
and services that are not authorized by
the Republic of Korea These contents
are not subject to review and regulation
of medical devices in Korea.


하이로닉 EU 웹사이트로 이동합니다.
EU(Europe) 웹사이트의 내용은 "CE MDD 또는 CE MDR" 허가승인된 품목의 제품 정보에 관한 것으로, 대한민국의 소비자 보호를 위한 국내법 의료기기의 광고 심의, 규제를 받지 않습니다.
It goes to the HIRONIC EU website.
The contents of the EU(European) web page are about product information of items approved for "CEMDD or CEMDR" in EU countries. They are not subject to advertising deliberation and regulation of domestic law medical devices for consumer protection in Korea.


하이로닉 중국어 웹사이트로 이동합니다.
중국어 웹사이트에는 중국내 판매제품으로
대한민국에서 허가하지 않은 장비 및 서비스
내용을 포함하고 있습니다. 해당 내용은
대한민국의 의료기기 광고 심의, 규제를 받지 않습니다.
正在移动到HIRONIC的中文官网。
在中问官网内介绍的产品只在中国销售的产品,
不一定是在韩国得到认可和服务内容。
针对这内容没有受到韩国医疗仪器广告审议限制。
