
New generation of skin care system

New generation of skin care system

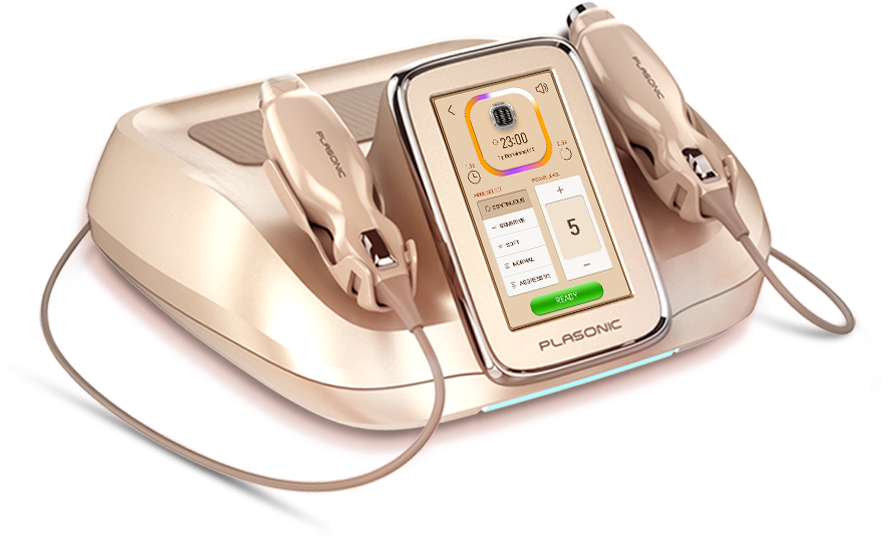
Incomparable solution delivery technology via plasma (PlaPass) and ultrasonic(SonoPass) in one system.
PlaPass technology stimulates solution delivery through effect. Then the skin with membrane potential effect, then SonoPass gives physical pressure on the particles and helps them to absorb into deep skin layer.
With the technology, aestheticians are able to provide. Wide range of customized premium programs with effect of DDS (Drug Delivery System)
99% of the material is made up of plasma in universe. Plasma may be the most abundant form of ordinary matter, although this hypothesis is currently tentative based on the existence and unknown properties. Plasma is one of the four fundamental states of matter along with solid, liquid and gas.

Recently, plasma has expended its application to the beauty market. Plasma ion is emitted as invisible size of 20 to 50 ㎛ which transfers the energy into the skin.
Solution are dissolved into the skin temporarily as the cell adhesion molecules break due by plasma effect.
Ultrasound (US) has an ever-increasing role in the delivery of therapeutic agents including genetic material, proteins, and chemotherapeutic agents.
Sonopass is an active form of transdermal delivery which enhances the transport of permeants, such as solution through cell membranes as a result of ultrasonic energy.

Plasonic PlaPass and SonoPass technologies allow
for the active ingredients of Plasonic Skin Booster Ampoule
to reach deeper layers of skin and create a new level
of synergy. Plasonic supplies
PLASONIC Ampoule supplies 35 highly concentrated active ingredients
deep into the skin that keep skin radiant and firm.

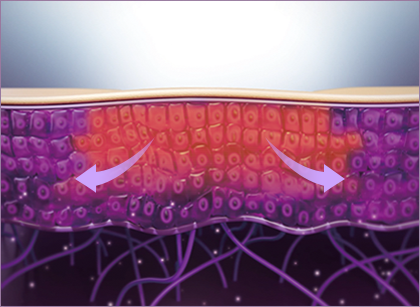
 PlaPass
PlaPass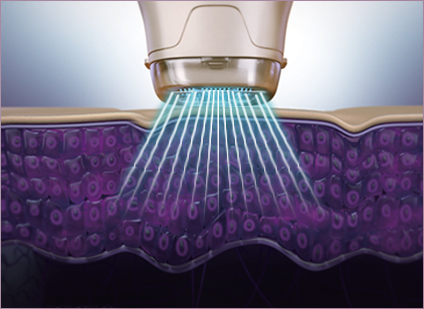
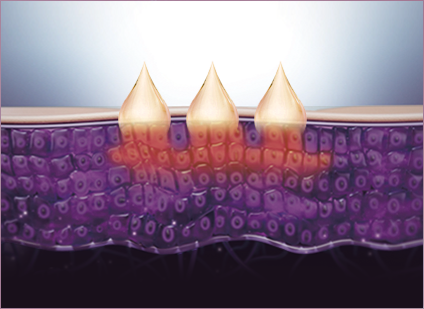

STEP 1 Ampoule boost with plasma technology
Plasma energy effectively creates tiny openings between cells for direct absorption of the Plasma Skin Booster Ampoule in the area undergoing PlaPass treatment.
 SonoPass
SonoPass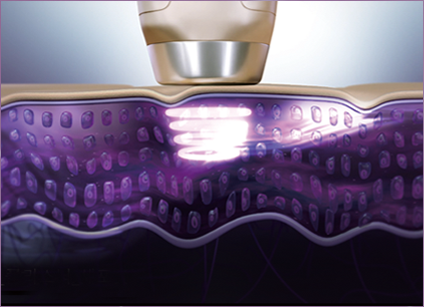

STEP 2 Additional ultrasonic application!
Stable application of ultrasonic waves at 3 MHz ensures even distribution and further absorption of ingredients to maximize hydration, firming and wrinkle-smoothing, pore tightening, and more synergizing effects for healthy, tight skin from the inside.
 Ampoule Effect
Ampoule Effect
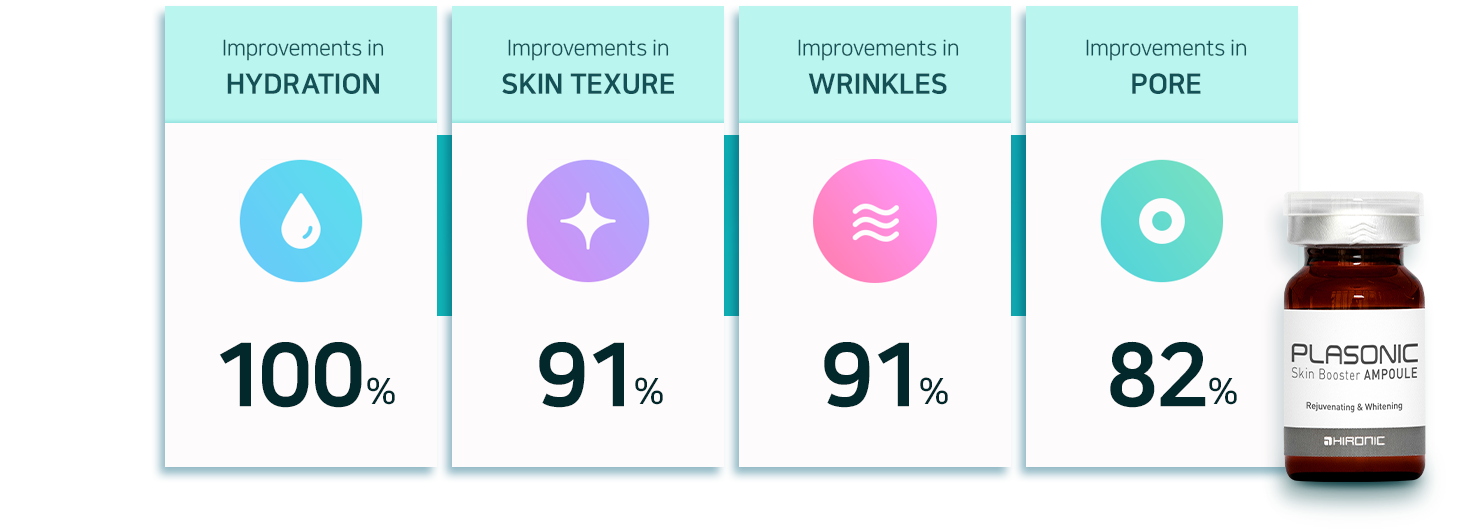
* The above results compare the values before and after application and obtained from the testers who showed improvements.
* Combined application of PLASONIC and Plasonic Skin Booster Ampoule / 4.16.2019~14.05 / 20~60 years 22 Females / OATC co.,Ltd. Skin Clinical Trial Center
* This device a manufactured good strictly offering cosmetic improvements. It is not a medical device, nor does it provide any medicinal benefits.
* The actual results of application may vary depending on the characteristics of an individual’s skin and other cosmetic products that are used together.

Helps deep miniaturization and nurturing of skin with low-molecular hyaluronic acid
and 17 amino acids to hydrate and activate cells.


Improves skin texture niacinamide for whitening and 17 vitamins for a naturally radiating tone,
along with hyaluronic and amino acids.
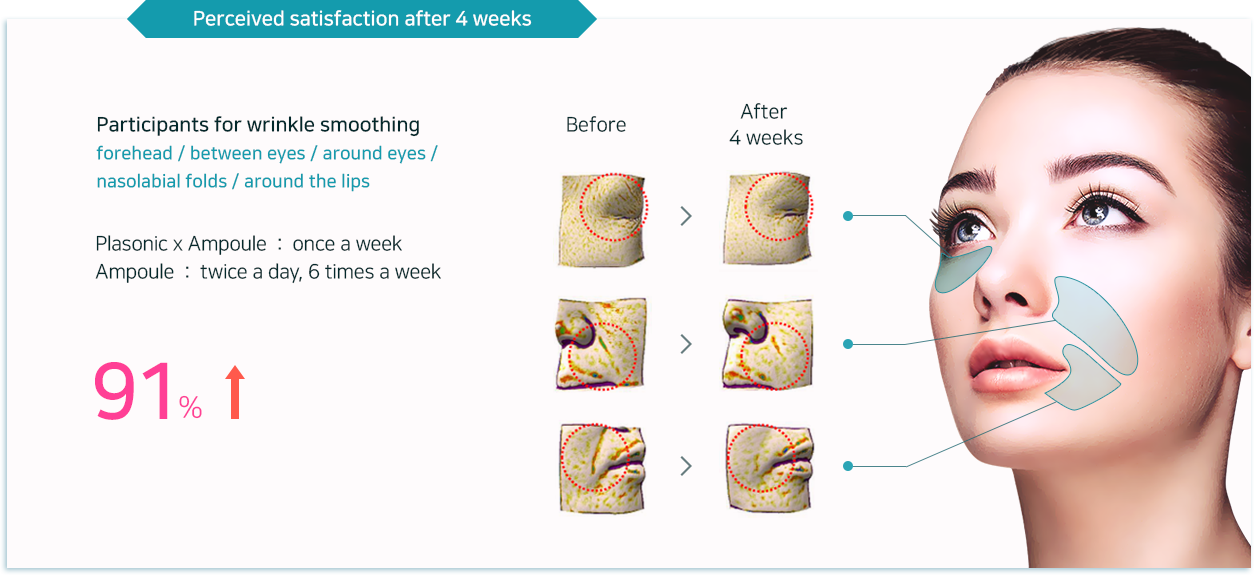
Adenosine with anti-wrinkle properties helps smoothing out wrinkles in five different areas
(forehead / between eyes / around eyes / nasolabial folds / around the lips)

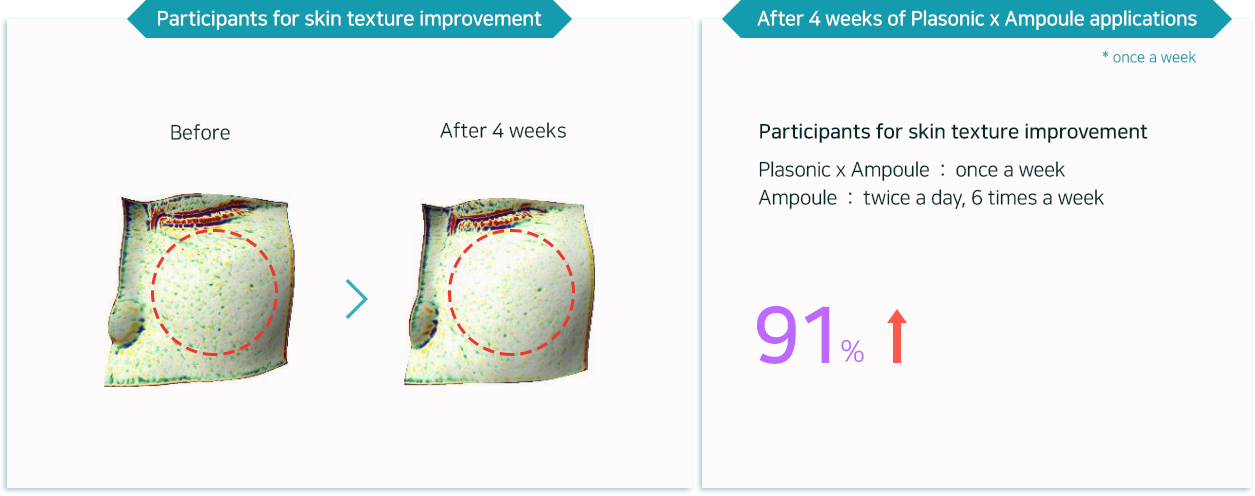
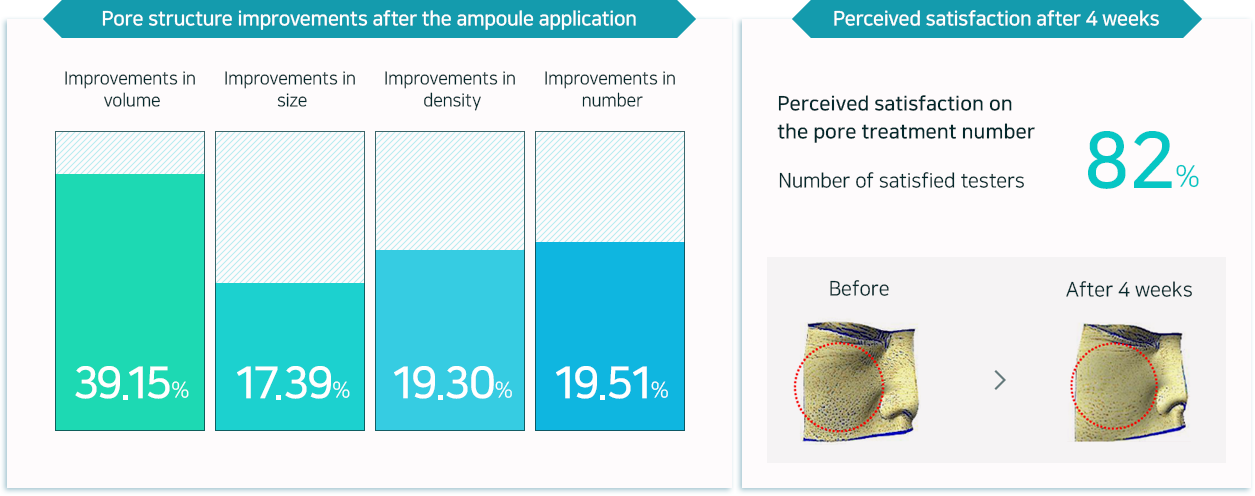
Provides four types of pore structure improvements for healthy, firm skin with tightening of pores in size and volume,
as well as reducing the number of visible pores and the density.
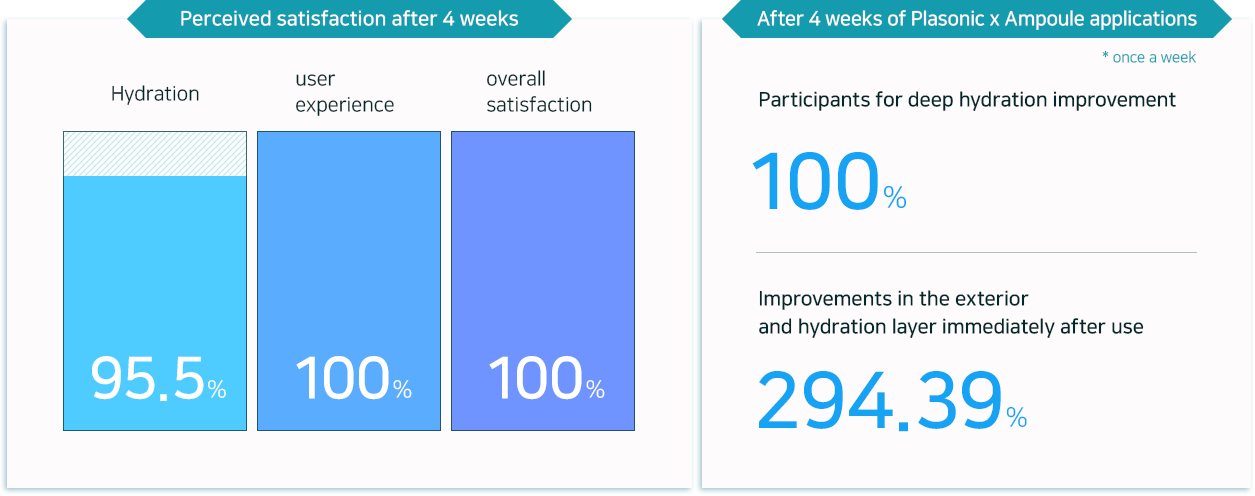
The results of the study showed that the effective ingredient permeability was 4.66 times higher than that of the control group and this shows that there is a synergy effect of maximizing the absorption effect of PLASONIC and skin improvement with Plasonic Skin Booster Ampoule of the 35 active ingredients.
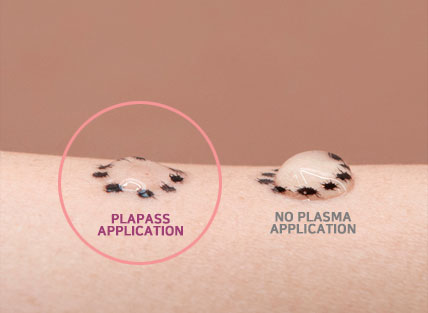
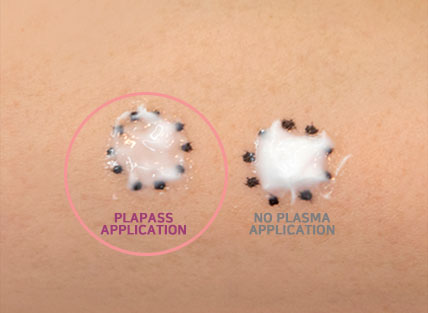
After plasma treatment, all types of ampoule through low and high viscosity, are highly absorbed within 30 seconds. Plapass sterilizes and stimulates the skin treatment process which leads to anti-bacteria, skin regeneration, DDSD and toning effect.
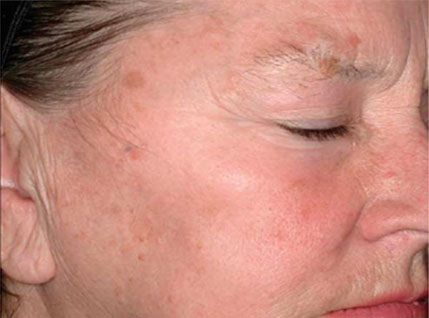
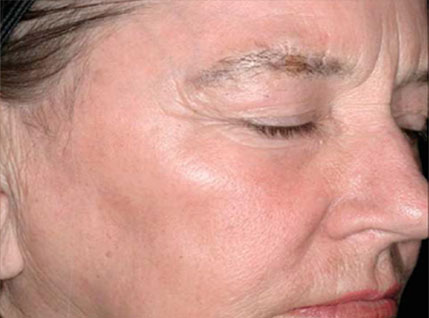
(Reference) Evaluation of Plasma Skin Regeneration Technology in Low-Energy Full–Facial Rejuvenation
Melissa A. Bole, MD; Kenneth A. Arndt, MD; Jefferey S. Dover, MD, FRCPC
Facial appearance before (A) and 3 month after (B) plasma skin regeneration,
with improvement in pigmentation and skin texture. Investigator-rated improvement on the 9-point 8 improvement in overall skin rejuvenation was 90%. This result also shows that plasma can effect skin toning and slight wrinkle care.
High Power Energy

PlaPass Square Tip

Long Lasting Tip
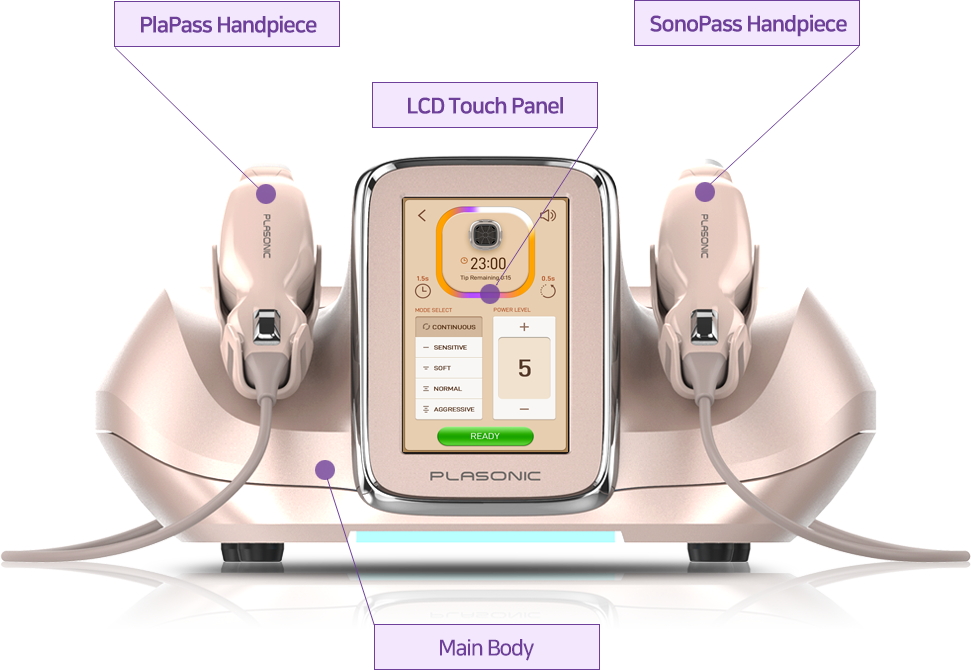
Plasonic Device Advantage

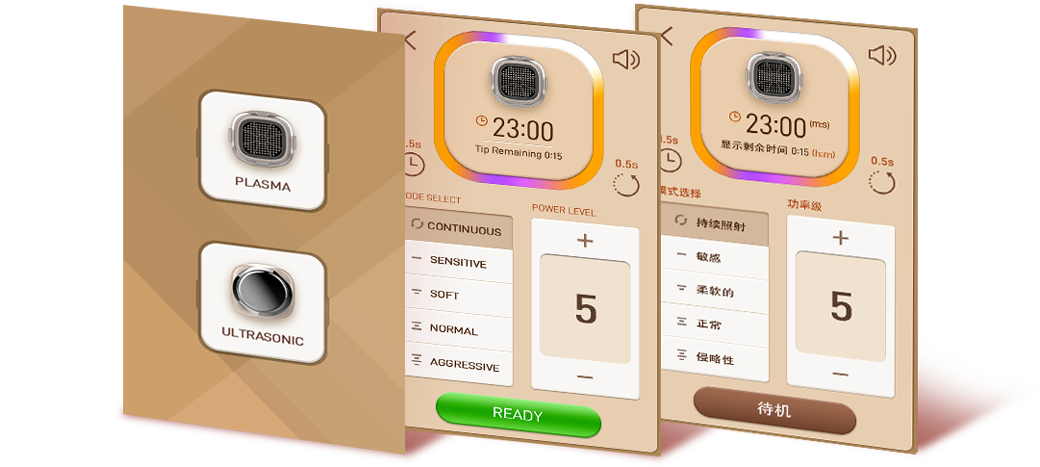
Intuitive Touch
Panel

User Friendly Device

Ergonomic
Hand-piece Design
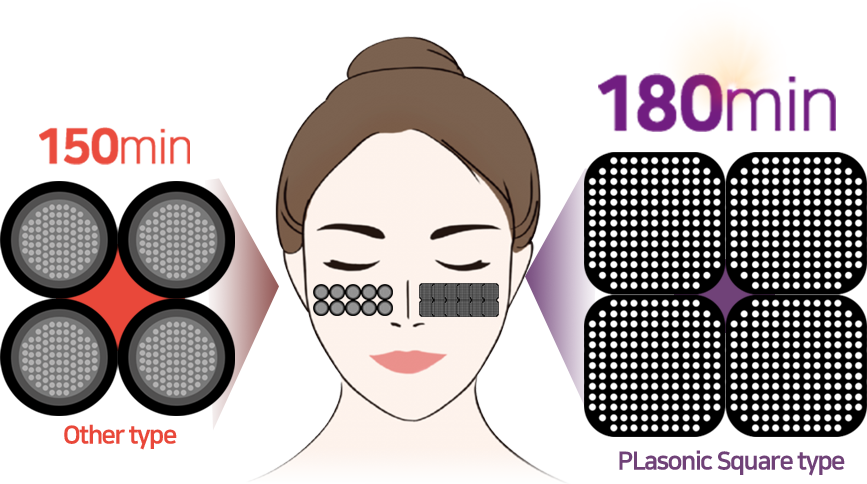
Square tip designed on consideration of minimize the energy loss
Plasma tip of PLASOSNIC is able to be used up to 3 hours (180 minutes) each. It lasts 20% longer than other tips Therefore, 15 more users can be treated with one tip, making it more efficient and affordable.
Multi language system,
Intuitive icon, and GUI system


Semi-automatic detachable
and bi-directional hand piece cradle


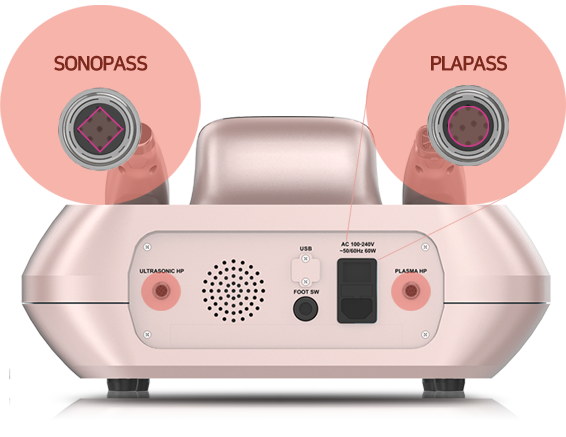
Different shapes of hand-piece
connectors to prevent misconnection

Dubai Derma 2019
Location : International Convention
Date : March 18 – March 20
HIRONIC participated at Dubai Derma 2019!
Regarding the exhibition, more than 200 visitors visited our booth and got many inquiries from 25 countries. Thanks to visitors, we could finish this exhibition very successfully, and it was a good opportunity to know the market trend and needs in the Middle East region and promote our new products such as Plasonic and Slimus. We sincerely hope to see you at Dubai Derma 2020 again.
Location : International Convention
Date : March 18 – March 20

HIRONIC participated at Dubai Derma 2019!
Regarding the exhibition, more than 200 visitors visited our booth and got many inquiries from 25 countries. Thanks to visitors, we could finish this exhibition very successfully, and it was a good opportunity to know the market trend and needs in the Middle East region and promote our new products such as Plasonic and Slimus. We sincerely hope to see you at Dubai Derma 2020 again.





KIMES 2019
Location : COEX, Seoul, Korea
Date : March 14 – March 17
HIRONIC participated at KIMES 2019!
HIRONIC successfully finished KIMES 2019 (35th Korea International Medical & Hospital quipment Show) held at the COEX Exhibition Center in Seoul from March 14th - 17th. KIMES is the largest medical equipment show in Korea and more than 70,000 visitors including 4,143 foreign visitors attended. Taking this opportunity, HIRONIC exhibited its main flagship products such as DOUBLO GOLD, MICOOL, SLIMUS, PLASONIC, ULTRA VERA, Q-FIT, and A-FIT. We were pleased to meet the medical professionals and share ideas with existing and potential partners. We are already looking forward to KIMES 2020 and welcome your visit.
Location : Seoul, Korea
Date : March 14 – March 17

HIRONIC participated at KIMES 2019!
HIRONIC successfully finished KIMES 2019 (35th Korea International Medical & Hospital quipment Show) held at the COEX Exhibition Center in Seoul from March 14th - 17th. KIMES is the largest medical equipment show in Korea and more than 70,000 visitors including 4,143 foreign visitors attended. Taking this opportunity, HIRONIC exhibited its main flagship products such as DOUBLO GOLD, MICOOL, SLIMUS, PLASONIC, ULTRA VERA, Q-FIT, and A-FIT. We were pleased to meet the medical professionals and share ideas with existing and potential partners. We are already looking forward to KIMES 2020 and welcome your visit.







ADD 2019
Location : WASHINGTON, D.C.
Date: March 1 - March 3
HIRONIC participated at AAD 2019!
HIRONIC attend AAD 2019 (American Academy of Dermatology) which is held at the Washington Convention Center from March 1st - 3rd, 2019. More than 18,000 doctors attended and, with this opportunity HIRONIC displayed its prime products such as SLIMUS, PLASONIC, Q-FIT, and A-FIT(USA FDA approved). We were glad to meet our current partners and also new potential partners and enjoyed the meetings with many medical professionals. HIRONIC is already looking forward to AAD 2020 to be held in Denver and welcomes your visit
Location : WASHINGTON, D.C.
Date: March 1 - March 3

HIRONIC participated at AAD 2019!
HIRONIC attend AAD 2019 (American Academy of Dermatology) which is held at the Washington Convention Center from March 1st - 3rd, 2019. More than 18,000 doctors attended and, with this opportunity HIRONIC displayed its prime products such as SLIMUS, PLASONIC, Q-FIT, and A-FIT(USA FDA approved). We were glad to meet our current partners and also new potential partners and enjoyed the meetings with many medical professionals. HIRONIC is already looking forward to AAD 2020 to be held in Denver and welcomes your visit








IMCAS 2019
Location : Paris, France
Date : January 31 – February 2
HIRONIC participated at IMCAS 2019!
IMCAS 2019 was another successful exhibition for HIRONIC! We had meaningful meetings with various medical professionals in field, and would like to thank everyone who visited our booth to share valuable time together. Not only that, we had a lecture about Doublo Gold and Plasonic, presented by Dr. Sabatier (Dermatologist, France) to provide clinical information about devices for users.
Location : Paris, France
Date : January 31 – February 2

HIRONIC participated at IMCAS 2019!
IMCAS 2019 was another successful exhibition for HIRONIC! We had meaningful meetings with various medical professionals in field, and would like to thank everyone who visited our booth to share valuable time together. Not only that, we had a lecture about Doublo Gold and Plasonic, presented by Dr. Sabatier (Dermatologist, France) to provide clinical information about devices for users.




EADV 2018
Location : Paris, France
Date : September 12 – September 16
HIRONIC will participate at EADV 2018!
EADV2018 will provide the best platform for all participants to boost and update their knowledge in dermatological science and technology through a variety of courses, workshops and lectures. Join HIRONIC to see innovative devices which includes our newly launched SLIMUS.
Location : Paris, France
Date : September 12 – September 16

HIRONIC will participate at EADV 2018!
EADV2018 will provide the best platform for all participants to boost and update their knowledge in dermatological science and technology through a variety of courses, workshops and lectures. Join HIRONIC to see innovative devices which includes our newly launched SLIMUS.



FACE 2018
Location : QEII Centre, London
Date : JUNE 14 – JUNE 16
HIRONIC participated at FACE 2018!
Hironic was able to successfully attend FACE 2018, UK’s premiere scientific conference featuring the latest clinical information, practical tips and updates in the field aesthetic treatment. It was our second launching show for SLIMUS and thankfully many doctors and professionals were interested in SLIMUS, and had done many demonstration treatments. For FACE 2018, HIRONIC displayed Doublo Gold, Micool-A, and SLIMUS. Once again, we highly appreciate your visit. See you soon!
Location : QEII Centre, London
Date : JUNE 14 – JUNE 16

HIRONIC participated at FACE 2018!
Hironic was able to successfully attend FACE 2018, UK’s premiere scientific conference featuring the latest clinical information, practical tips and updates in the field aesthetic treatment. It was our second launching show for SLIMUS and thankfully many doctors and professionals were interested in SLIMUS, and had done many demonstration treatments. For FACE 2018, HIRONIC displayed Doublo Gold, Micool-A, and SLIMUS. Once again, we highly appreciate your visit. See you soon!
하이로닉 영문 웹사이트로 이동합니다.
글로벌 웹사이트에는 수출용 제품으로
대한민국에서 허가하지 않은 장비 및 서비스
내용을 포함하고 있습니다. 해당 내용은
대한민국의 의료기기 광고 심의, 규제를 받지 않습니다.
It goes to the HIRONIC English website.
The Global Web Site contains equipment
and services that are not authorized by
the Republic of Korea These contents
are not subject to review and regulation
of medical devices in Korea.


하이로닉 EU 웹사이트로 이동합니다.
EU(Europe) 웹사이트의 내용은 "CE MDD 또는 CE MDR" 허가승인된 품목의 제품 정보에 관한 것으로, 대한민국의 소비자 보호를 위한 국내법 의료기기의 광고 심의, 규제를 받지 않습니다.
It goes to the HIRONIC EU website.
The contents of the EU(European) web page are about product information of items approved for "CEMDD or CEMDR" in EU countries. They are not subject to advertising deliberation and regulation of domestic law medical devices for consumer protection in Korea.


하이로닉 중국어 웹사이트로 이동합니다.
중국어 웹사이트에는 중국내 판매제품으로
대한민국에서 허가하지 않은 장비 및 서비스
내용을 포함하고 있습니다. 해당 내용은
대한민국의 의료기기 광고 심의, 규제를 받지 않습니다.
正在移动到HIRONIC的中文官网。
在中问官网内介绍的产品只在中国销售的产品,
不一定是在韩国得到认可和服务内容。
针对这内容没有受到韩国医疗仪器广告审议限制。
