
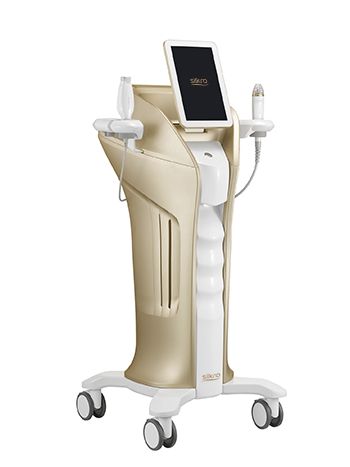
Hironic, a company specializing in beauty medical devices, announced on 8th that its all-in-one radio frequency device ‘Silkro (Korean model name ‘Gentlo’)’ has obtained U.S. Food and Drug Administration (FDA). After being certified with European CEMDD in July last year.
"Silkro" is an all-in-one radio frequency solution that not only causes coagulation of cell tissue but also relieves pain by penetrating micro needles into the skin or transferring radio frequency energy into the skin through electrodes on the handpiece. The device has total of four handpieces: RM (RF Micro Needle), RN (RF Needle), RC (RF Circle), and RV (RF Venus).
Among the four handpieces, the RM handpiece needles penetrates the skin and emits radio frequency energy into the skin. The needle tip comes in 25pin or 49pin needles which give users a choice to use in wide or smaller facial area.
The needle comes in two types: insulated, which energy is transferred only to the target point, and non-insulated, which energy is transferred from the entire needle, allowing the procedure to be designed according to the patient's concerns or skin characteristics.
In addition, Silkro is equipped with Treatment Information System (TIS), which provides convenience with functions that manage the history of procedures such as the type of handpiece, energy value, and number of times used automatically when patient information is entered.
An official from Hironic said, "Silkro is certified with CE MDD certification, and now has obtained U.S. FDA approval. This is a start for Hironic's radio frequency technology to expand in global market". He also added that with FDA approval, the company will target the North American market, which accounts for more than 40% of the global beauty and medical device market, as well as achieve high sales and long-term corporate growth."

하이로닉 영문 웹사이트로 이동합니다.
글로벌 웹사이트에는 수출용 제품으로
대한민국에서 허가하지 않은 장비 및 서비스
내용을 포함하고 있습니다. 해당 내용은
대한민국의 의료기기 광고 심의, 규제를 받지 않습니다.
It goes to the HIRONIC English website.
The Global Web Site contains equipment
and services that are not authorized by
the Republic of Korea These contents
are not subject to review and regulation
of medical devices in Korea.


하이로닉 EU 웹사이트로 이동합니다.
EU(Europe) 웹사이트의 내용은 "CE MDD 또는 CE MDR" 허가승인된 품목의 제품 정보에 관한 것으로, 대한민국의 소비자 보호를 위한 국내법 의료기기의 광고 심의, 규제를 받지 않습니다.
It goes to the HIRONIC EU website.
The contents of the EU(European) web page are about product information of items approved for "CEMDD or CEMDR" in EU countries. They are not subject to advertising deliberation and regulation of domestic law medical devices for consumer protection in Korea.


하이로닉 중국어 웹사이트로 이동합니다.
중국어 웹사이트에는 중국내 판매제품으로
대한민국에서 허가하지 않은 장비 및 서비스
내용을 포함하고 있습니다. 해당 내용은
대한민국의 의료기기 광고 심의, 규제를 받지 않습니다.
正在移动到HIRONIC的中文官网。
在中问官网内介绍的产品只在中国销售的产品,
不一定是在韩国得到认可和服务内容。
针对这内容没有受到韩国医疗仪器广告审议限制。
